Bipolar Disorder Treatment Pipeline Shows Strong Momentum as 20+ Pharma Companies in the Race | DelveInsight
The bipolar disorder clinical trial landscape is evolving with 25+ therapies, namely, KarXT (BMS), RAP 219 (Rapport Therapeutics), ABBV-932 (AbbVie Inc.), ABX-002 (Autobahn Therapeutics), ALTO-100 (Alto Neuroscience), CB03-154 (Zhimeng Biopharma), 4MT 2001 (4M THERAPEUTICS), and others, which are in different phases of bipolar disorder clinical trials.
New York, USA, Sept. 11, 2025 (GLOBE NEWSWIRE) -- Bipolar Disorder Treatment Pipeline Shows Strong Momentum as 20+ Pharma Companies in the Race | DelveInsight
The bipolar disorder clinical trial landscape is evolving with 25+ therapies, namely, KarXT (BMS), RAP 219 (Rapport Therapeutics), ABBV-932 (AbbVie Inc.), ABX-002 (Autobahn Therapeutics), ALTO-100 (Alto Neuroscience), CB03-154 (Zhimeng Biopharma), 4MT 2001 (4M THERAPEUTICS), and others, which are in different phases of bipolar disorder clinical trials.
DelveInsight’s 'Bipolar Disorder Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for bipolar disorder across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the bipolar disorder domain.
Key Takeaways from the Bipolar Disorder Pipeline Report
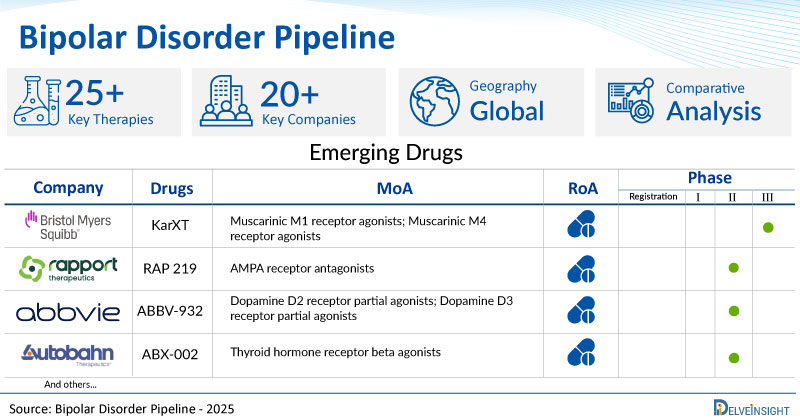
- DelveInsight’s bipolar disorder pipeline report depicts a robust space with 20+ active players working to develop 25+ pipeline bipolar disorder drugs.
- Key bipolar disorder companies such as Bristol-Myers Squibb, Alzamend Neuro, Inc., Reviva Pharmaceuticals, Rapport Therapeutics, NRx Pharmaceuticals, LB Pharmaceuticals, Lundbeck, AbbVie, Autobahn Therapeutics, Alto Neuroscience, Zhimeng Biopharma, 4M THERAPEUTICS, and others are evaluating new bipolar disorder drugs to improve the treatment landscape.
- Promising pipeline bipolar disorder therapies, such as KarXT, AL001, RP5063, RAP 219, NRX-101, LB 102, Aripiprazole 2-month injectable, ABBV-932, ABX-002, ALTO-100, CB03-154, 4MT 2001, and others, are in different phases of bipolar disorder clinical trials.
- Notable MoA in clinical trials include Muscarinic M1 receptor agonists; Muscarinic M4 receptor agonists, AMPA receptor antagonists, Dopamine D2 receptor partial agonists; Dopamine D3 receptor partial agonists, Thyroid hormone receptor beta agonists, Brain-derived neurotrophic factor modulators, KCNQ2 potassium channel stimulants; KCNQ3 potassium channel stimulants, Glycogen synthase kinase 3 beta inhibitors, and others.
- In August 2025, NRx Pharmaceuticals, Inc. announced that the US Food and Drug Administration (FDA) had granted Fast Track designation to NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression.
- In June 2025, 4M Therapeutics Inc. announced positive results from completed GLP safety studies for its lead asset, 4MT2001. The full results reinforce the encouraging safety profile observed to date and demonstrate a significant therapeutic window for safe dosing. There were no adverse effects on the cardiovascular system at any dose level.
- In May 2025, Alzamend Neuro, Inc. announced the dosing of the first patient of its first Phase II clinical study of AL001 in healthy human subjects. This study follows the successful development of a novel head coil by Tesla Dynamic Coils BV, a key component of the clinical trial. The study in healthy human subjects will serve as a baseline and assist Alzamend determine the best path forward in Alzheimer’s, BD, MDD and PTSD patients by demonstrating AL001’s targeted effectiveness and reduced systemic side effects.
- In March 2025, Alzamend Neuro, Inc. announced its plans to initiate a highly anticipated phase II clinical study of AL001 for treatment of patients with BD, which is expected to commence in the third quarter of 2025. This study follows the successful completion of a head coil by Tesla Dynamic Coils BV, a key component of the clinical trial.
- In January 2025, Autobahn Therapeutics, announced the initiation of a Phase II clinical trial evaluating ABX-002, its highly potent, oral, thyroid hormone beta receptor (TRβ) selective agonist, as an adjunctive treatment in adults with bipolar depression. The Phase II trial aims to establish biological and clinical proof-of-concept for ABX-002 in bipolar depression and will inform Autobahn’s further clinical development strategy.
- In October 2024, AbbVie announced a new discovery, co-development and license agreement to advance novel targets for the potential treatment of neuropsychiatric conditions. This collaboration expands upon the success of nearly two decades of partnership on central nervous system (CNS) projects, including globally launched products such as cariprazine (VRAYLAR® / REAGILA®) and the discovery of investigational drug candidate ABBV-932 for the treatment of bipolar depression and generalized anxiety disorder.
- In October 2024, Autobahn Therapeutics announced the clearance of an investigational new drug (IND) application by the US Food and Drug Administration (FDA) to support initiation of a Phase II trial of ABX-002 as an adjunctive treatment for patients with bipolar depression.
- In July 2024, Alto Neuroscience, Inc. announced that the Company has been awarded USD 11.7 million by the Wellcome Trust to advance the clinical development of lead candidate ALTO-100 through a Phase IIb study in patients with bipolar depression characterized by a cognitive biomarker.
Request a sample and discover the recent advances in bipolar disorder drugs @ Bipolar Disorder Pipeline Report
The bipolar disorder pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage bipolar disorder drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the bipolar disorder clinical trial landscape.
Bipolar Disorder Competitive Landscape
Leading companies, including Bristol-Myers Squibb, Alzamend Neuro, Inc., Reviva Pharmaceuticals, Rapport Therapeutics, NRx Pharmaceuticals, LB Pharmaceuticals, Lundbeck, AbbVie, Autobahn Therapeutics, Alto Neuroscience, Zhimeng Biopharma, 4M THERAPEUTICS, and others are currently active in the bipolar disorder competitive landscape.
Among these, Bristol-Myers Squibb is currently evaluating its candidate KarXT in the late stage of development, i.e., phase III. On the other hand, drugs such as RAP 219 (Rapport Therapeutics), ABBV-932 (AbbVie Inc.), ABX-002 (Autobahn Therapeutics), ALTO-100 (Alto Neuroscience), and others are in the mid-stage of development. Whereas, CB03-154 (Zhimeng Biopharma), 4MT 2001 (4M THERAPEUTICS), and others are still in early stages of development.
Learn more about the emerging bipolar disorder therapies @ Bipolar Disorder Clinical Trials
Bipolar Disorder Therapeutics Assessment
The bipolar disorder pipeline report proffers an integral view of the emerging bipolar disorder therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Bipolar Disorder Pipeline Report
- Coverage: Global
- Bipolar Disorder Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Bipolar Disorder Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Bipolar Disorder Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Bipolar Disorder Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Bipolar Disorder Therapeutics Assessment By Mechanism of Action: Muscarinic M1 receptor agonists; Muscarinic M4 receptor agonists, AMPA receptor antagonists, Dopamine D2 receptor partial agonists; Dopamine D3 receptor partial agonists, Thyroid hormone receptor beta agonists, Brain derived neurotrophic factor modulators, KCNQ2 potassium channel stimulants; KCNQ3 potassium channel stimulants, Glycogen synthase kinase 3 beta inhibitors
- Key Bipolar Disorder Companies: Bristol-Myers Squibb, Alzamend Neuro, Inc., Reviva Pharmaceuticals, Rapport Therapeutics, NRx Pharmaceuticals, LB Pharmaceuticals, Lundbeck, AbbVie, Autobahn Therapeutics, Alto Neuroscience, Zhimeng Biopharma, 4M THERAPEUTICS, and others.
- Key Bipolar Disorder Pipeline Therapies: KarXT, AL001, RP5063, RAP 219, NRX-101, LB 102, Aripiprazole 2-month injectable, ABBV-932, ABX-002, ALTO-100, CB03-154, 4MT 2001, and others.
Dive deep into rich insights for new bipolar disorder treatments, visit @ Bipolar Disorder Drugs
Table of Contents
| 1. | Bipolar Disorder Pipeline Report Introduction |
| 2. | Bipolar Disorder Pipeline Report Executive Summary |
| 3. | Bipolar Disorder Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Bipolar Disorder Clinical Trial Therapeutics |
| 6. | Bipolar Disorder Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Bipolar Disorder Pipeline: Late-Stage Products (Phase III) |
| 8. | Bipolar Disorder Pipeline: Mid-Stage Products (Phase II) |
| 9. | Bipolar Disorder Pipeline: Early-Stage Products (Phase I) |
| 10. | Bipolar Disorder Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Bipolar Disorder Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Bipolar Disorder Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the bipolar disorder pipeline therapeutics, reach out @ Bipolar Disorder Therapeutics
Related Reports
Bipolar Disorder Epidemiology Forecast
Bipolar Disorder Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted bipolar disorder epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Bipolar Depression Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key bipolar depression companies, including Allergan (AbbVie), Gedeon Richter, Sunovion Pharmaceuticals (Sumitomo Dainippon Pharma), Intra-Cellular Therapies, NeuroRx, Sunovion (Sumitomo Dainippon Pharma), Lundbeck, Otsuka Pharmaceutical, Celon Pharma, COMPASS Pathways, among others.
Major Depressive Disorder Market
Major Depressive Disorder Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key MDD companies, including Takeda Pharmaceuticals, Forest Laboratories, Otsuka Pharmaceuticals, Janssen Research & Development, Axsome Therapeutics, AbbVie, SAGE Therapeutics, Minerva Neurosciences, Luye Pharma, Relmada Therapeutics, BioLite Inc., VistaGen Therapeutics, Praxis Precision Medicines, Intra-Cellular Therapies, Neurocrine Biosciences, Arrivo Bioventures, Sirstei Pharmaceuticals, Alto Neuroscience, Chase Therapeutics, Neumora Therapeutics, Inc., BlackThorn Therapeutics, Inc., Otsuka Pharmaceutical Co., Ltd., Fabre-Kramer Pharmaceuticals, Novartis, among others.
Treatment-Resistant Depression Market
Treatment-Resistant Depression Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key treatment-resistant depression companies, including Navitor Pharmaceuticals, Supernus Pharmaceuti, Novartis, Relmada Therapeutics, Beckley Psytech, COMPASS Pathways, Axsome Therapeutics, Celon Pharma, among others.
Postpartum Depression Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key postpartum depression companies, including Sage Therapeutics, Biogen, Marinus Pharmaceuticals, Lipocine, Brii Biosciences Limited, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
